Transforming Water into Ice instantly
Md Islam
The City college of New York
English 21007
Instructor: Thomas Barber
04/11/2019
Abstract:
This experiment will measure the nucleation effect as different refrigeration times of purified water has an effect on successfully turning water into ice instantly. Three experimental groups were created with each given different freezing time and the results were recorded. The group with 165 minutes was found to be most successful. The result supported the hypothesis as the water require specific freeze time for it to instantly turn into ice.
Introduction:
The purpose of this experiment is to determine the freezing time required for instant ice. Instance ice is the process of turning water into ice within seconds. Ice is widely used by many people around the world for daily activities. Ice is more desired in the summer season to cool objects. Some of the uses of ice may include cooling down during the summer, using ice as part of beauty routine remove gum from clothing/ hair, preventing a stain from setting into clothes, [1] and muscle recovery after intense physical activity. Ice can be bought from retail stores or simply made at home. The process in which ice is made is fairly simple. The process involves taking water into a container and placing into the freezer for few hours and would be ready to use. The only problem is that it takes hours for the liquid to turn into ice.
The freezing point of water is 32 degrees Fahrenheit or 0 degrees Celsius [2]. However, the time it takes to freeze the water may depend on many factors. Some of which may include the size of the container in which the water is placed. [2]. It will take less time to freeze a small bottle of water compared to a big bottle of water. Another factor may also include the surface area of the container, this is simply due to “ice starts to freeze from outside in [2]”. And most importantly, the temperature of the freezer. Most home freezers have preselected the temperature to 0 degrees Fahrenheit or -17.78 degrees Celsius. With this preset temperature, the estimated time for water to freeze is about three to four hours[2].
What happens when there is no ice in an emergency situation? It turns out that ice can be made instantly with very less effort and time. In order to turn water/ any liquid to solid ice, it needs nucleus which allows it to form small crystals and eventually ice. The website Britannica explains the technical term for this process is called nucleation [3]. Britannica states,
“nucleation, the initial process that occurs in the formation of a crystal from a solution”[3]. Through doing so, the liquid which contains very small numbers of ions, [3] and atoms soon align in a pattern of small crystal-like solids [3]. And over time, nucleation continues to occur on the small particle crystals and eventually forms ice[3]. Placing water long enough into the freezer will make it ice. Therefore, it is safe to hypothesize that, one should take the water out the freeze at the correct time such that 2 hour and 45 minutes to successfully turn water into ice instantly.
Objectives:
This experiment will measure nucleation effect as different refrigeration times of purified water has effect on successfully turning water into ice instantly. Three group of water bottles was created, each group gets different freezing time. Afterward, the groups will be compared to see the success rate of instant ice from each group.
Materials:
- 15 unopened purified bottles of water (500 ml)
- Home Freezer (0 degrees Fahrenheit)
- Flat surface (tables).
- Ice cubes (15 small size).
- Container to place the ice cubes
Procedure:
- Separate the 15 bottles into three groups (group 1 with 5 bottles, group 2 containing 5 bottles, and group 3 containing 5 bottles).
Figure1: Group the bottles

- Place all the bottles (unopened purified) of water into the freezer around 120 to 180 minutes.
Figure 2: Place the bottles into the freezer

- Take out group 1 (5 bottles) after 150 minutes (2 hour and 30 mins) of freezing.
- Take out group 2 (5 bottles) after 165 minutes (2 hour and 45 minutes) of freezing.
- Take out group 3 (5 bottles ) after 180 minutes ( 3 hours) of freezing.
- After taking out each group from the freezer, gently open the bottle and pour the water into the ice.
Figure 3: Pouring water into the ice

- Record the number of bottles successfully turned into ice from each group. This will identify the success rate from each group. For instance, 2 out of 5 bottles success at instant ice, the success rate would be 40%.
Results:
Table 1: Success rate of instant ice at different freezing time.
| Groups | Number of bottles | Freezer temperature (degrees Fahrenheit) | Freezing time (Minutes) | Success rate (percentage %) |
| Group 1 | 5 bottles | 0 degrees Fahrenheit | 150 minutes | 60 % |
| Group 2 | 5 bottles | 0 degrees Fahrenheit | 165 minutes | 80% |
| Group 3 | 5 bottles | 0 degrees Fahrenheit | 180 minutes | 20% |
As shown in the table 1 above, three groups were created, each group containing 5 unopened water bottles. The freezing temperature (degrees Fahrenheit) was kept constant throughout the experiment. The only variable that was changed during this experiment was the freezing time. Group 1 was given a freezing time of 150 minutes, group 2 of 165 minutes, and group 3 of 180 minutes freezing time. The success rate of each of the three groups was recorded. Group 1 had a 60% success rate, group 2 with 80% success rate, and group 3 with a 20% success rate.
Figure 4: Success rate of instant ice at different freezing time.
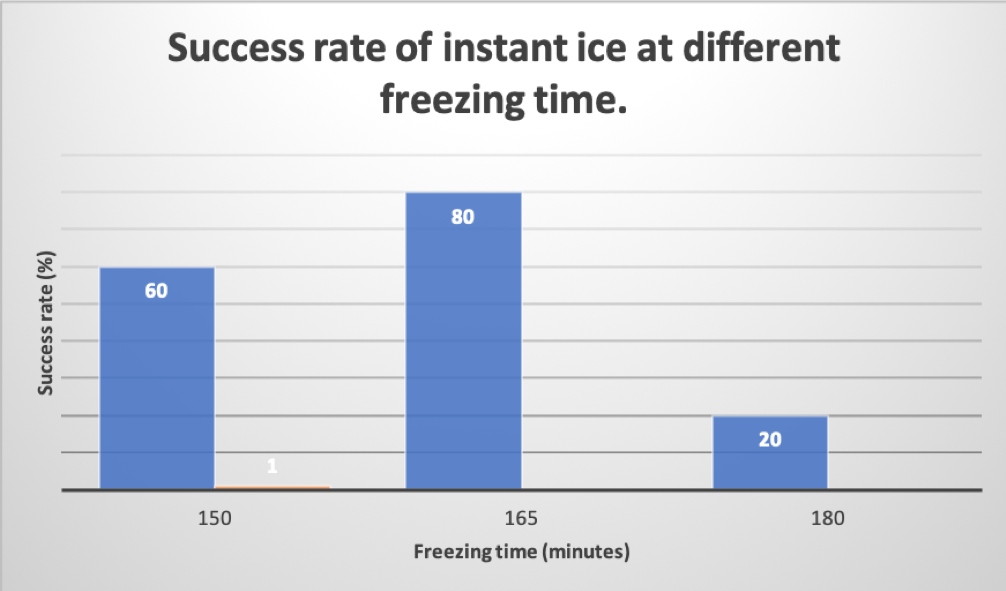
This is a visual representation of Table 1. On the x-axis labeled (freezing time in minutes). On the y-axis labeled (success rate in percentage %). Figure 4 represents the relationship between freezing time and success rate of water turning into ice instantly.
Analysis:
The data of this experiment support the hypothesis, as the water require specific freeze time for it to instantly transform into ice instantly. As shown in Table 1 and Figure 4, freezer time of 165 minutes has a higher chance of instant ice success with 80%. Other freezing time may be less effective due to simply that the water may have not formed enough crystals to turn ice. Alternately, if given too much freezing time, the water may have turned to ice before even taking it out the freezer. 180 minutes freezing time had less success rate (20%) comparing to the other two groups because the water has frozen due to a longer time in the freezer. While this result is satisfying and expected, there may be some flaws to it, and may not be completely accurate. Some of alternate explanation may include, the room temperature as it may have made some the water colder or hot depending on the environmental temperature. Others may include, upon taking out the water bottle from the freezer, holding the bottle too tight. This is so because the water has the potential to turn ice if applied enough force. Also to consider human errors, such as incorrectly timing the freeze time, or failed to follow the procedure. Some future direction for this experiment to consider includes: upon placing the water into the freezer, place the bottles sideways instead of straight upward. Also, it is important to ensure that the environment in which this experiment is being done is not too hot, it may have an effect on the outcome.
Reference List
[1] “9 uses for ice cubes | 1 Million Women,” 9 uses for ice cubes | 1 Million Women. [Online]. Available: https://www.1millionwomen.com.au/blog/9-amazing-uses-ice-cubes/. [Accessed: 11-Apr-2019].
[2] “How Long Will It Take for My Ice Cubes to Freeze? Answers to All Your Ice-Making Questions,” NewAir. [Online]. Available: https://www.newair.com/blogs/learn/how-long-for-ice-cubes-to-freeze. [Accessed: 11-Apr-2019].
[3] T. E. of E. Britannica, “Nucleation,” Encyclopædia Britannica, 03-Feb-2014. [Online]. Available: https://www.britannica.com/science/nucleation. [Accessed: 11-Apr-2019].

